ocrevus start up form
Date of birth Prescribers first name. These infusion side effects can happen up to 24 hours after getting a dose of Ocrevus.

Most Impressive Drug Launch Roche S Ocrevus Biopharma Dive
Is this a new start or continuation of therapy.
:max_bytes(150000):strip_icc()/the-immune-system-and-multiple-sclerosis-5208473_final-fc7a43bf81994d93a087d77183d08839.jpg)
. Genentech can start helping you when page 4 of this form is submitted by you or your doctors office in one of the following ways. Once youve prescribed OCREVUS enroll your patients in OCREVUS Access Solutions. These infusion reactions can happen for up to 24 hours after your infusion.
Prescription Enrollment Form. Inform patients that infusion reactions can occur up to 24. Swelling of the throat.
A representative from OCREVUS Access Solutions or your. Certain side effects are possible after an Ocrevus infusion. RMS and PPMS and their open-label extensions up to.
Ocrevus ocrelizumab Fax completed form to 8883021028. Prior Authorization Form for. It is important that.
Examples of infusion side. Ocrevus ocrelizumab Medication Precertification Request Aetna Precertification Notification Phone. It is a one-time registration completed by the.
1-888-267-3277 For Medicare Advantage Part B. Access the OCREVUS Start Form and learn more about the assistance Genentech offers for your OCREVUS ocrelizumab patients. When possible you should receive any non-live vaccines at least 2 weeks before you start treatment with.
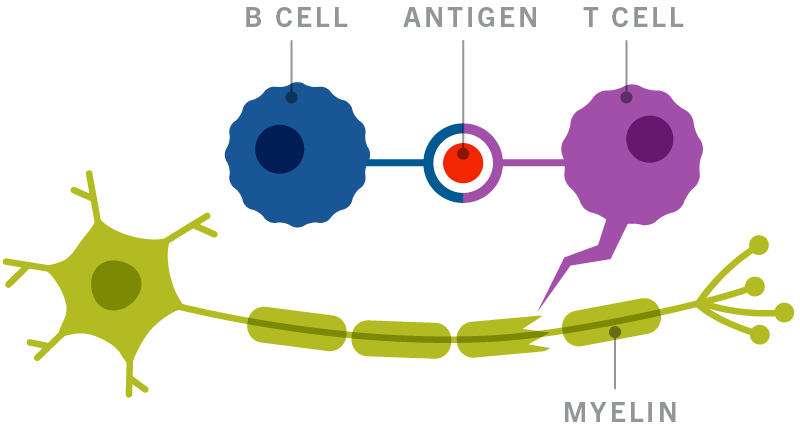
Relapsing forms of multiple sclerosis MS to include clinically isolated syndrome relapsing-remitting disease and active secondary. OCREVUS is a prescription medicine used to treat. Ocrevus ocrelizumab injection is a preservative-free sterile clear or slightly opalescent and colorless to pale brown solution supplied as a carton containing one 300.
If your patient has already begun treatment with drug samples of Ocrevus please choose new start of therapy. These infusion reactions can happen for up to 24 hours after your infusion. OCREVUS Start Form for ocrelizumab Who May See and Use My PII I authorize Genentech andor Genentech Patient Foundation to i use my PII for the purpose of facilitating my access.
This form is used to initiate the EFT registration process when the practice chooses not to use check reimbursements.

The Future Of Multiple Sclerosis

Ocrevus Side Effects Cost Uses And More

Ocrevus Ocrelizumab Results For Rms Relapsing Ms

Multiple Sclerosis Two Decades Of Progress The Lancet Neurology

Roche S Ocrevus 2 Hour Infusion Time Gets Eu Approval For Ms Treatment S P Global Market Intelligence

Ectrims2022 Targeting Ebv Key In Progressive Ms Experts Ata188 May Be Game Changing Treatment For Non Active Forms Multiple Sclerosis News Today
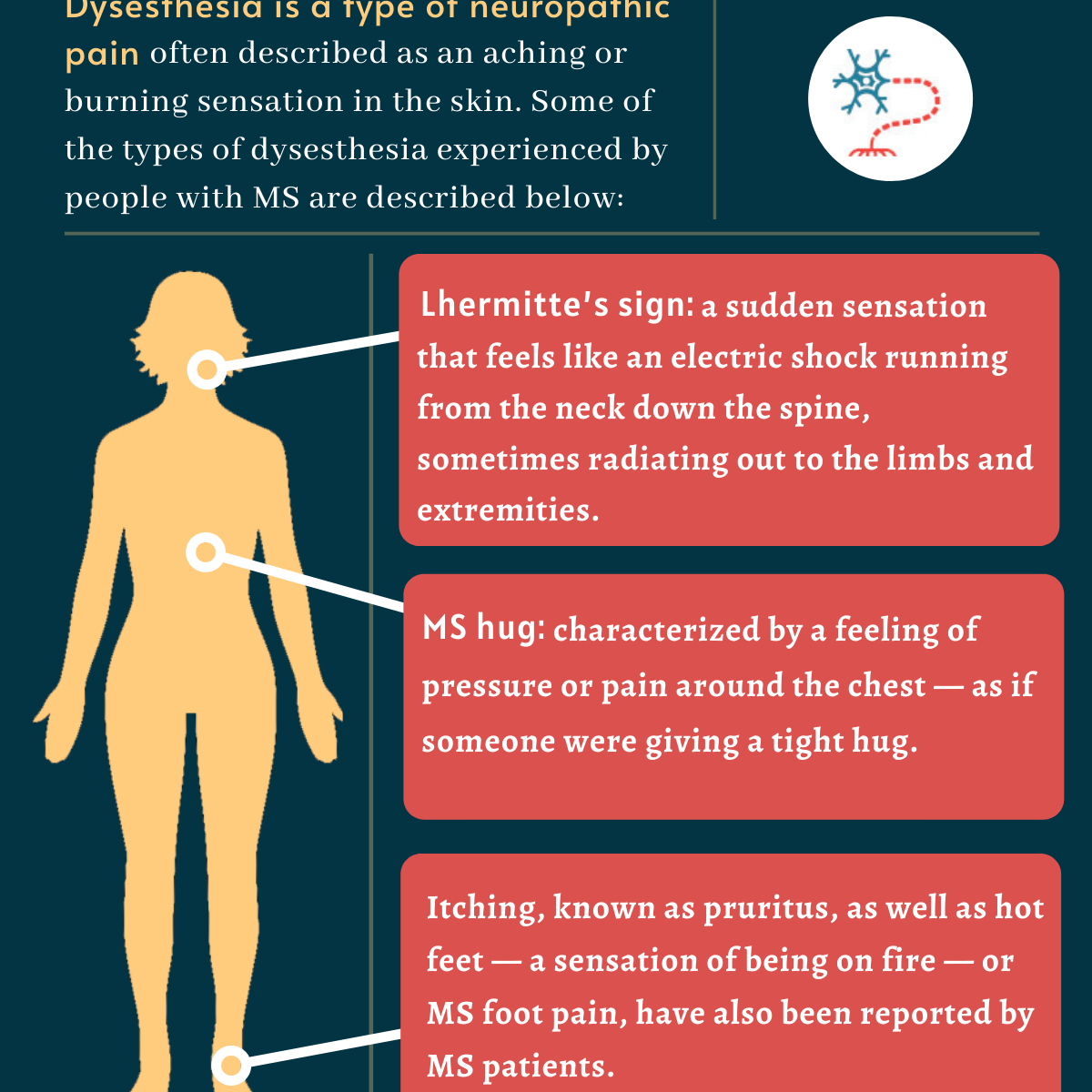
Dysesthesia In Ms Feeling Causes And Treatment Multiple Sclerosis News Today
Progressive Ms Research Multiple Sclerosis Ms International Federation
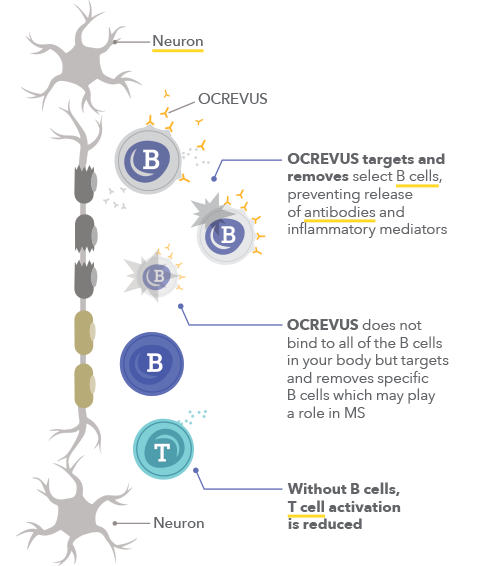
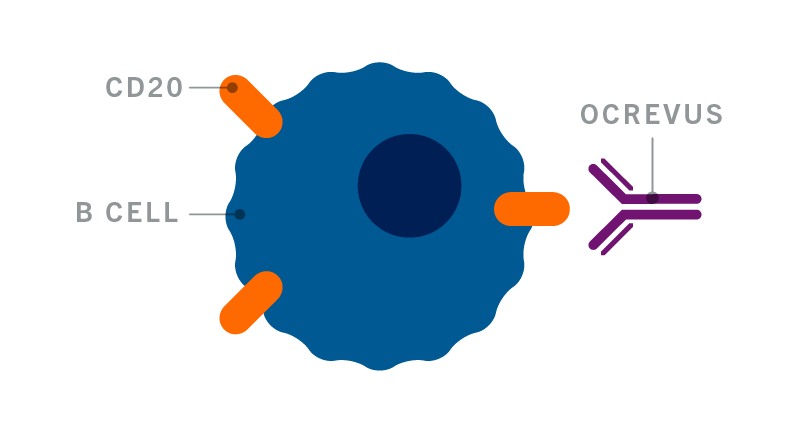
How Does Ocrevus Work Get On With Life
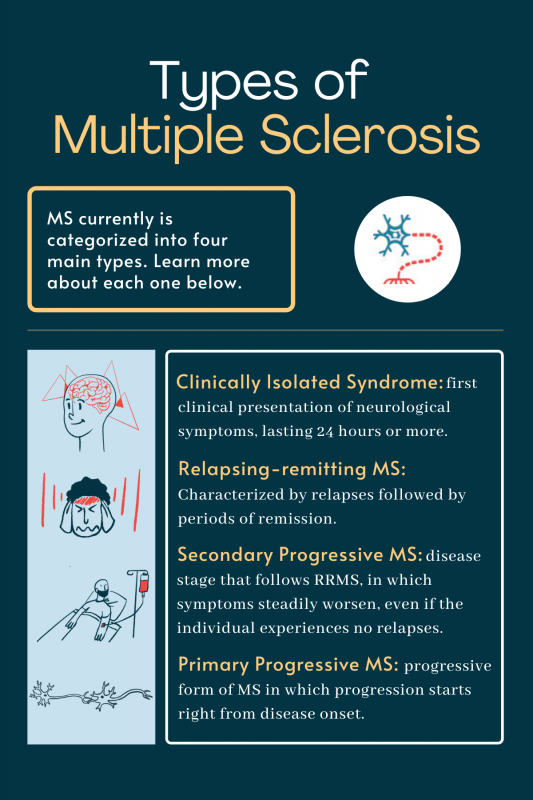
What Are The Different Types Of Ms Multiple Sclerosis News Today
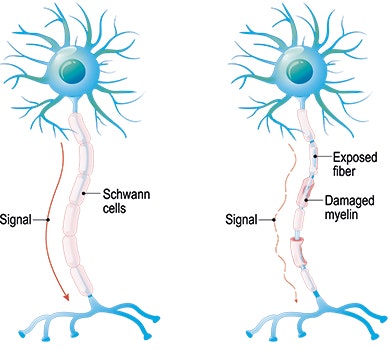
Multiple Sclerosis Ms Symptoms Progression Diagnosis Etc Stiwell

Submit Print Or Download Ocrevus Forms Documents Ocrevus Access Solutions

Humana Continuity Of Care Form Fill Out Sign Online Dochub
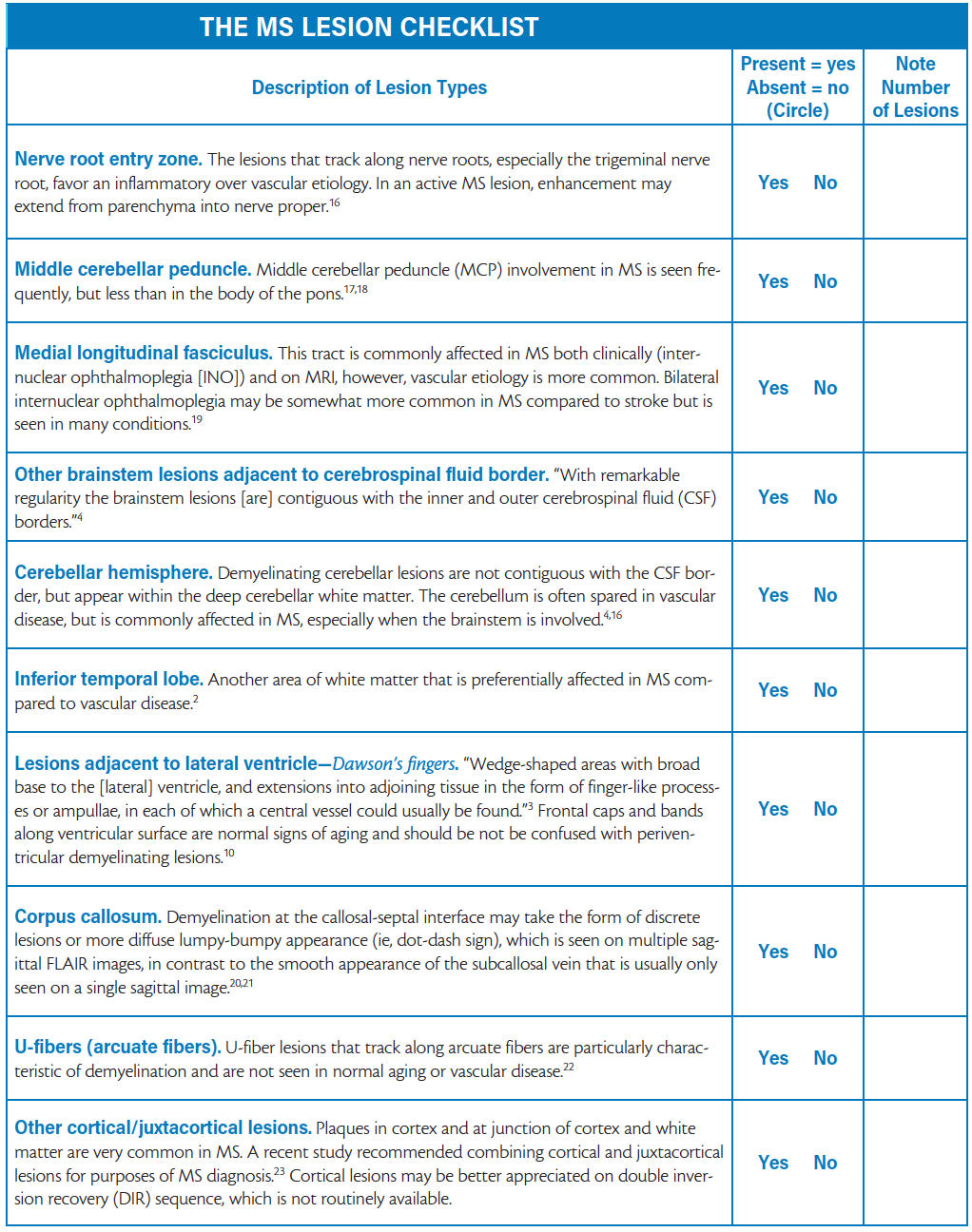
The Multiple Sclerosis Lesion Checklist Practical Neurology

Ocrevus Start Form Fill Online Printable Fillable Blank Pdffiller
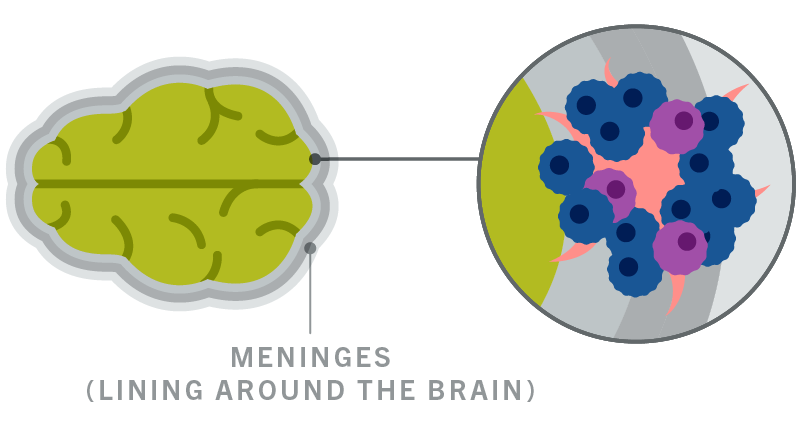
:quality(90)/)